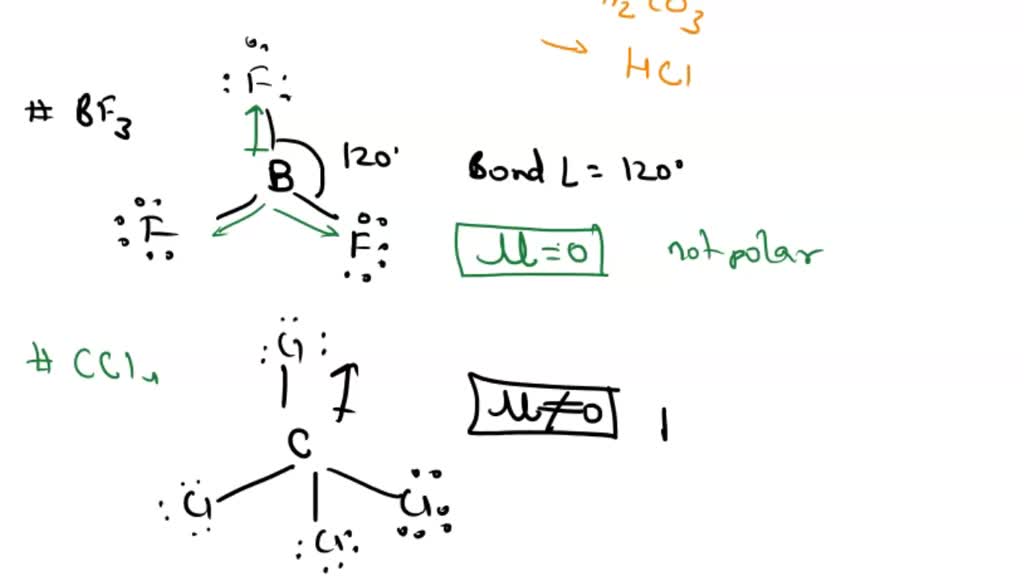
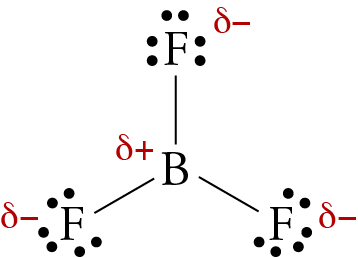
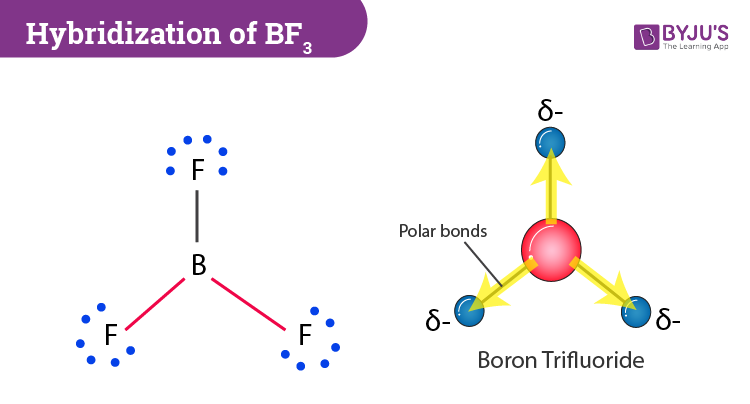
All three of the boron-fluorine single bonds in bf3 are polar. in which direction should the polarity - brainly.com
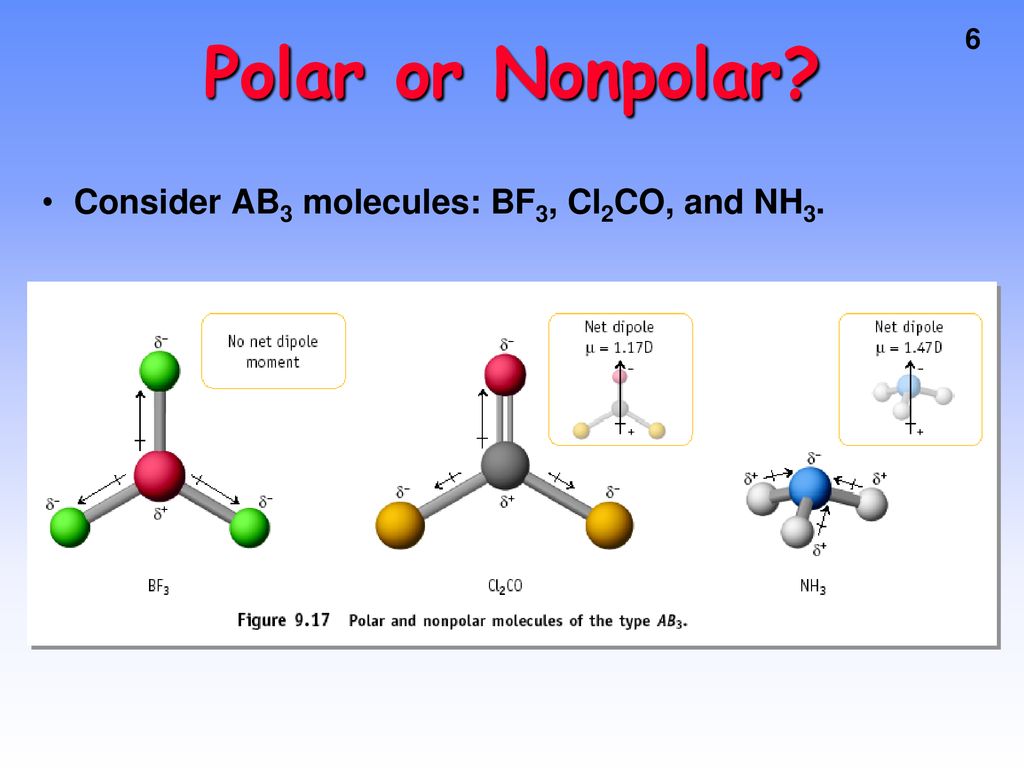
Boron trifluoride (BF3) is a nonpolar molecule, whereas ammonia (NH3) is a polar molecule. The difference in polarities is related to the fact that :

Boron Trifluoride BF3 is a non polar molecule whereas ammonia NH3is a polar molecule. The difference in polarity is related to the fact that